Novel Therapeutic Formulations
Technology Cluster
Radiopharmaceutical Kits
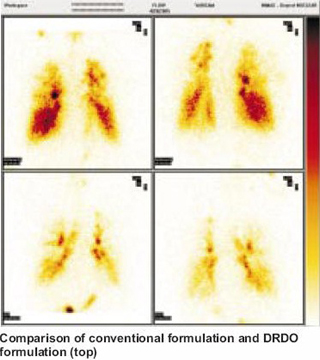
Novel Therapeutic Formulations ITAD Formulations Using conventional respiratory formulations, only five per cent of the inhaled drug penetrates to the depths in the lung towards the alveoli. The therapeutic effect on the lungs is therefore limited. For this reason, only a few respiratory formulations of salbutamol and steroids have been developed though a number of diseases afflict the alveoli. DRDO has taken an early initiative in the field and has developed five novel respiratory formulations. These include beta- agonists and nitric oxide donor drugs that release nitric oxide at the site of action (alveoli). Using scintigraphy, their deep penetration into the lungs (5-15 times more than the conventional methods) has been confirmed. Their therapeutic benefit has also been confirmed in human beings as a part of zero-phase human trial ahead of regular clinical trials. Their utility has been shown in cases with pulmonary hypertension (reduction in pulmonary blood pressure by 20-40 per cent as measured by ECHO), in increasing oxygen transfer to the blood (in hypoxic hypoxemia), in improvement of lung perfusion and in reducing cardio- pulmonary stress. Enhancement in blood oxygen and lung perfusion was confirmed by pulse-oximetry and lung perfusion scintigraphy, respectively. These formulations are likely to be a new form of treatment in asthma, chronic bronchitis & ARDS. In all these conditions, the present treatment is inadequate and there is a further scope of improvement. Multicentric trials are now planned in collaboration with the Army, Indo-Tibetan Border Police, Patel Chest Institute and RBTB Hospital. Partners from pharmaceutical industry include Jamia Hamdard and Cipla India Ltd.
Transdermal Local Vasodilator Formulations
DRDO has developed the concept of treating peripheral vasculopathies using locally administered vasodilators. Nuclear medicine methods have helped to confirm and quantify the vasodilator action in human. Salbutamol and nitroglycerine ointments produced by DRDO have now entered phase-2 human trial after regulatory clearance. Government of Himachal Pradesh is partnering DRDO in this collaborative venture. The product will be useful in mild cold injuries and diabeticlage-related peripheral vasculopathy.