Magnetic Resonance Perfusion Imaging
Technology Cluster
Perfusion ideally represents a mean of measuring blood delivery to tissues at the capillary level. It depends on physiological parameters such as blood pressure, velocity of flow, capillary network, and also on microvascular anatomy, histology, blood microcirculation, and the blood- tissue exchange rates. Cerebral capillary flow rate has been estimated to be of the order of 1.5 mm/s. The application of perfusion imaging or microvascular hemodynamic imaging techniques to the brain is likely to have a significant impact on the study of ischemic disease and stroke, in the initial evaluation and in assessment of reperfusion related to interventional trials, as well as characterisation and understanding of intracranial pathophysiology.
In conventional radionuclide studies, cerebral blood flow (CBF) measurement is achieved by a freely-diffusible tracer such as nitrous oxide or by single photon emission computed tomography (SPECT). Here, the tracer concentrates in the tissue. The magnetic resonance (MR) perfusion technique offers the advantage of non-ionising raDIATion with potential for improved spatial and temporal resolution with minimal invasion. By simultaneous use of paramagnetic contrast material and ultra fast imaging, it is possible to study the hemodynamics of normal and diseased human brain. In MR perfusion imaging, the first pass of an intravascular paramagnetic non-diffusible
contrast agent through a region of interest is studied. While in normal MR angiography, the image voxel contains 100 per cent of flowing elements, in perfusion imaging it is only 2-4 per cent, which makes the technique very delicate. In human brain, the first pass of contrast agent bolus is for about 15 s, which demands ultra fast imaging in order to adequately sample the signal-time curve. The MR signal is converted into concentration values by assuming a linear dependence between the contrast agent concentration and T 2 relaxation rate. Relative cerebral blood volume (rCBV), which is a measure of the amount of capillaries in a volume element, can be determined by numerically integrating the concentration-time curve during first pass of the contrast agent bolus. The relative cerebral blood flow (rCBF) can be determined if the arterial input function of the contrast agent is known.
Imaging of brain perfusion using SPECT or positron-emission tomography (PET) techniques do not give morphological information about the brain, which limit their clinical use in routine practice. The MR perfusion imaging provides an opportunity for image perfusion in the same session as conventional and diffusion- weighted MR imaqing.
Clinical Applications
Clinical applications of perfusion imaging can be broadly categorised as:
- Stroke and cerebrovascular diseases
- CNS neoplasms
- White matter disease
- Non-anatomic disorders
- Seizures
- Psychiatric disorders
- Dementias and other neuro-degenerative disorders
Ischemic Strokes
Studies combining diffusion and perfusion studies have suggested that an acute stroke (detected by diffusion weighted imaging) surrounded by hypoperfused zone (detected by perfusion imaging) could be at risk of enlargement. Acute perfusion and diffusion imaging lesion volumes correlate with the clinical outcome. The potential to achieve quantitative perfusion data can be used to differentiate the ischemic penumbra from the central infarcted core of a non-viable tissue. Combining perfusion and diffusion imaging seems to be a particularly effective method to assess the early hemodynamic changes in acute ischemic strokes.
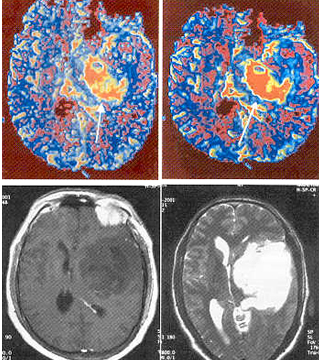
Tumor Imaging
Blood volume mapping is potentially useful in grading primary tumors. The MR perfusion blood volume mapping correlates strongly with PET findings in most of the studies. High-grade gliomas has been found to be heterogeneous in such mapping studies compared with low-grade lesions, making grading of gliomas possible with rCBV mapping. The MR perfusion imaging also offers powerful means for assessing pathophysioloqical changes associated with blood-tumor barrier. These imaging techniques hold immense potential to characterise and monitor intervention in disease processes of brain and promise to expand the reach of neuro-radiology into the fundamentals of clinical problems, including tumor biology and pathophysiology, stroke patho- physiology and recovery, epilepsy, dementia, and many others.